Lawyer proposes a new legal framework for reviewing FDA’s refusal to regulate lethal injection drugs.
One mission of the U.S. Food and Drug Administration (FDA) is to ensure that drugs used on humans are safe. What does this mean for drugs that states use to administer death?
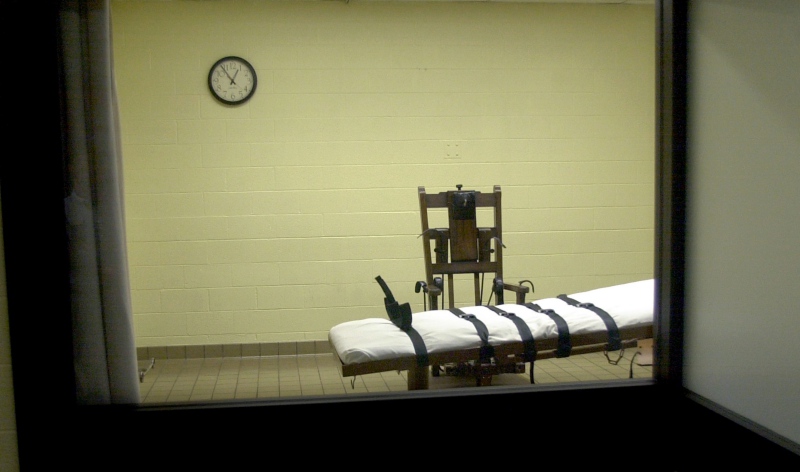
Federal and state governments execute most incarcerated people on death row by lethal injection. When states have a difficult time obtaining lethal injection drugs, executions slow.
Yet FDA has routinely refused to regulate drugs used in lethal injections, and its refusal has long been shielded from judicial review. Even if FDA is legally obligated to regulate lethal injection drugs, the U.S. Supreme Court has held that agencies have discretion over when to enforce the law—or when not to enforce it. Given the high stakes of lethal injection, lawyer Andrew Shi proposes a new standard of judicial review that would allow courts to review FDA’s refusal to regulate lethal injection drugs.
Shi explains that, historically, states with capital punishment obtained lethal injection drugs from domestic pharmaceutical companies subject to FDA regulations. Although causing death is not an FDA approved purpose, the drugs used in executions met FDA safety standards when used for approved purposes such as sedation and pain management. Over the last decade, however, domestic companies have increasingly refused to distribute their otherwise life saving or improving drugs to correctional facilities for use in executions. When states have then attempted to import lethal injection drugs, the Supreme Court has required FDA to confiscate unregulated foreign drugs. As a result, states have increasingly faced lethal injection drug shortages.
Rather than reevaluate their use of the death penalty, some state officials turned to local drug compounders for lethal injection drugs. Drug compounders are local shops where pharmacists mix drugs for individual patients with unique needs. Because compounded drugs are mixed on an individual basis, they do not undergo the typical FDA approval process and are regarded by some experts as less safe than FDA approved drugs.
Shi claims that FDA has the statutory authority to regulate compounded drugs, but he acknowledges that the agency refuses to exercise this authority. In the absence of federal oversight, states are largely responsible for licensing and overseeing drug compounders.
States often exercise this authority lightly. Some states legislatures have passed laws to shield compounders from the oversight of state medical and pharmacy boards. Almost half of all states allow, or require, correctional facilities to hide the identity of compounded lethal drug suppliers to ensure a continued supply of the drugs.
The use of these often unregulated compounded drugs for lethal injection has led to botched executions in recent years. In 2014, one person spent two hours “gasping and snorting” before the drugs took their final effect. In Texas, five men administered lethal drugs were able to report that they felt like they were burning “inside out” as they passed.
In a 2019 opinion, the U.S. Department of Justice concluded that FDA does not have authority to regulate lethal injection drugs. The Justice Department cited the Supreme Court’s reasoning in FDA v. Brown & Williamson in which the Court held that Congress did not intend to give FDA authority to regulate cigarettes as drug devices under the Food, Drug, and Cosmetic Act (FDCA). Because the FDCA requires FDA to deem drugs and devices safe for use to be on the market, such a grant of authority would have required the agency to ban cigarettes. But a ban would have contradicted other statutes Congress had passed, according to the Court. In its opinion, the Justice Department similarly argued that if FDA had authority over lethal injection drugs, the agency would have to ban them for this use because FDA could never deem these drugs safe for use in human executions. The Justice Department claims that, like in Brown & Williamson, this result must take FDA’s authority over lethal drugs outside the scope of what Congress intended when it passed the FDCA.
Shi finds the Justice Department’s argument unpersuasive. Unlike the tobacco legislation in Brown & Williamson, Congress has not passed laws that demonstrate its intent to keep lethal injection drugs outside of FDA authority. In addition, the FDCA authorizes FDA to regulate “new” drugs. Although the FDCA does not explicitly classify compounded lethal drugs as new drugs, Shi argues that this conclusion is unavoidable based on the text of the statute. The FDCA defines new drugs as any drug that “is not generally recognized…as safe and effective.” Shi also notes that one federal court has held that, when compounders create otherwise approved drugs for unapproved or off-label purposes, the result is a new drug. Because drugs used in executions are not approved for the purpose of causing death, Shi concludes that they fall under the FDCA definition of new drugs.
Furthermore, Shi explains that Congress authorized FDA to regulate compounded drugs in the Food and Drug Administration Modernization Act. Although some compounded drugs are exempt from FDA regulation under the Act, Shi explains that these exceptions only apply to compounded drugs created for patients with valid prescriptions. Because doctors in virtually every state refuse to issue prescriptions to execute, Shi concludes that lethal injection drugs are not exempt from FDA’s authority to regulate compounded drugs under the Act.
But even if, as Shi argues, FDA does have the authority to regulate compound lethal injection drugs, the Supreme Court does not currently require the agency to exercise that authority. The Court has ruled that agency inaction is presumed unreviewable, reasoning that agency officials, rather than judges, are best positioned to determine how to use limited resources to meet overall enforcement responsibilities.
Shi explains that when reviewing agency inaction, courts only look to cases in which the agency has failed to implement the overall purpose of the statute. Because most agency inaction is not that broad, courts rarely review agency inaction under this current framework.
Given the gravity of the issue, Shi proposes a new legal standard he calls “discrete look” for courts to apply. Under the discrete look standard, courts could review an agency’s refusal to enforce a particular section of the law, rather than limiting judicial review of agency nonenforcement to instances where the agency fails to enforce the overall statute.
To prevent judicial review of nonenforcement from becoming too sweeping, Shi argues that this test should only apply when an agency refuses to take enforcement action against a private party and when no other regulator has authority over the same issue. According to Shi, both of these prerequisites are met in the case of FDA’s refusal to regulate compounded lethal injection drugs.
If courts adopt Shi’s proposed standard of review, individuals on death row could sue FDA over its refusal to regulate compounded lethal drugs. Shi argues that Congress has clearly granted FDA the power to regulate these drugs, and the agency should not be able to escape this grave responsibility. And once FDA asserts, or is forced to assert, its statutory authority, the agency could ban lethal injection drugs altogether, effectively ending lethal injections and the death penalty in the United States as it has come to be known.



