FDA approves first over-the-counter continuous glucose monitor for U.S. patients.
Two million people living with diabetes lack health insurance. But access to health insurance predicts whether individuals with diabetes will receive high-quality diabetes care.
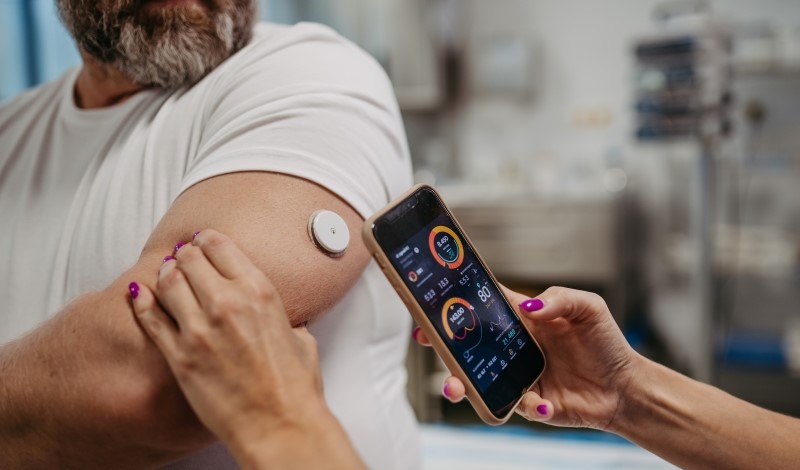
Earlier this year, the U.S. Food and Drug Administration (FDA) took action to confront this problem. The agency approved the first over-the-counter continuous glucose monitor, known as Stelo, for adults with diabetes who do not use insulin. Stelo, a sensor about the size of a quarter worn on the belly or arm and paired with a smartphone application, is aimed at helping people track and understand their blood sugar levels.
FDA approves medical devices such as Stelo through a process known as 510(k) review. During this process, a manufacturer must show that the device’s intended use, design, and performance is “substantially equivalent” to a product already available in the market. 510(k) review allowed Stelo to be approved for over-the-counter use by comparing it to its predecessor, also named Stelo, which required a prescription.
As routine part of diabetes self-management, glucose measurement allows individuals to adjust their lifestyle and prevent dangerous drops or spikes. Most people with diabetes have for years regulated their glucose levels with fingerstick checks, requiring them to prick their fingertips with a small needle to obtain a blood sample. But FDA’s approval of Stelo will enable more individuals to make diabetes treatment decisions without a fingerstick test.
FDA approved the original Stelo as a prescription device in 2017. As a Class 2 medical device, which poses moderate risk to patients, Stelo needed to clear clinical trials proving that the device is safe and accurate. The FDA reported that clinical trials found that Stelo’s glucose readings matched actual blood sugar levels and device-related side effects were minimal, such as local skin irritation and discomfort.
Barriers to obtaining Stelo as a prescription device, however, hindered its use. For individuals enrolled in public health insurance plans, such as Medicare and Medicaid, obtaining continuous glucose monitors has been difficult because the device is not covered in most states. Individuals without insurance could not access the device at all.
By removing the prescription requirement and approving Stelo as an over-the-counter device, the FDA now allows anyone with diabetes to get the device.
Jeff Shuren, FDA Director for Center for Devices and Radiological Health, emphasized that Stelo’s over-the-counter approval enhances health accessibility by providing people with “information about their health, regardless of their access to a doctor or health insurance.”
The information obtained from a continuous glucose monitoring device can be more accurate than fingerstick readings, which only provide a measurement of blood sugar level at the moment the blood sample was obtained. Factors, such as, eating new foods, exercise, stress, and alcohol consumption, can temporarily affect glucose levels even for people with well-controlled diabetes symptoms. Fingerstick readings cannot provide a complete picture of how blood sugar levels fluctuate throughout the day or in response to lifestyle changes, making it difficult to recognize potential causes of dips or spikes.
By contrast, continuous glucose monitors such as Stelo, supply users with real-time information about their blood sugar levels on their phones. The data gathered by Stelo allows people with diabetes to modify their diet or lifestyle and pinpoint potential causes of irregularities.
Real-time blood sugar level measurements can offer life-saving benefits for diabetes management by delaying or avoiding hospitalizations for complications such as amputations, kidney failure, or death. For example, studies show that people with diabetes who use continuous glucose monitors experience reduced hypoglycemia, a condition that occurs when the level of blood glucose drops below a certain amount. They also experience reductions in average blood glucose—or A1C.
Despite the FDA’s approval of Stelo’s for over-the-counter sales, the device still comes with a high price tag. The cost depends on frequency of testing, but on average, fingerpick test strips cost about $120 a month without insurance. By contrast, Stelo sensors cost over $300 a month plus an additional $300 for a new transmitter every three months. Given that nearly 30 percent of diabetes patients already neglect to monitor glucose levels because of cost, Stelo will remain financially unobtainable for many.
Some health care advocates urge federal and state regulators to reduce the costs of glucose monitoring technology by focusing on pharmacy benefit managers, third parties responsible for negotiating drug price discounts with manufacturers. These negotiations often result in higher out-of-pocket costs for continuous glucose monitors, regardless of an individual’s insurance coverage.
In addition, FDA’s clearance of Stelo for over-the-counter use opens the door for future devices that enable “glucose curious” individuals to monitor health data without a prescription. Today, consumers generally demand “wearables” that provide them with information on how their blood sugar reacts to certain workouts and foods. But currently, FDA has yet to approve a smartwatch that can measure blood glucose levels.
Micheal B. Natter, clinical assistant professor at New York University Grossman School of Medicine, reportedly suggests that the device could possibly be used as a preventative tool for individuals with prediabetes or to help detect undiagnosed diabetes. That said, people without diabetes may misinterpret glucose values, especially after meals when it is normal to experience blood sugar spikes.
Last month, Stelo sensors became available without a prescription for the first time.



