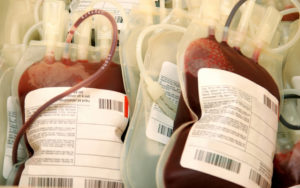
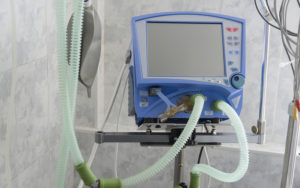
Federal Focus Shifts to COVID-19 Treatments
FDA releases guidance on using blood from recovered COVID-19 patients to treat new cases.
FDA Relaxes Rules on Ventilators for COVID-19
Responding to the coronavirus outbreak, a federal agency relaxes requirements on medical device manufacturers.
Will FDA Guidance Hasten Testing for COVID-19?
In the face of a massive viral outbreak, a federal regulator issues unprecedented guidance on validating COVID-19 testing.