Challenging Drug Patents to Lower Prices
The FTC cracks down on improperly listed drug patents, challenging over 100 patent listings.
The Bounded Triumph of Health Care Ballot Initiatives
Medicaid expansion ballot initiatives show state referenda can expand health care access—but they have their limits.
Cutting Corners on COVID-19 Treatments?
Scholars analyze FDA’s emergency use authorizations during the COVID-19 pandemic.
HHS Selects Drugs for Medicare Price Negotiation
Biden Administration introduces price regulation to the pharmaceutical industry.
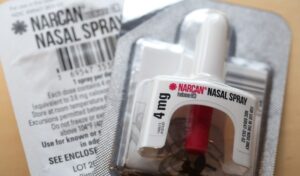
Cracking Down on Overdose Deaths
FDA approves first over-the-counter treatment to combat the opioid overdose epidemic.
Drug Safety, or Overregulation of Reproductive Health?
Scholar calls for FDA to remove stringent restrictions on medication abortion.
Underrepresented, Overmedicated, and Misdiagnosed
Experts contend that FDA clinical trials fail to ensure adequate consideration of women’s health needs.
The Borders of the U.S. Health Care System
Scholars explore regulatory reforms to expand noncitizens’ access to health care.
Regulating ESG Disclosure
Experts explore the evolving sphere of ESG regulation in light of greater calls for corporate accountability.
A Game of Inches for Youth Concussion Regulation
Experts explore the impact of concussion legislation on adolescent health and education.
Getting Disinformation Right
In this week’s Saturday Seminar, scholars explore the challenges and potential for regulating disinformation.