An Intersectional Approach to Regulating Women’s Health
Scholar calls for regulators to correct the dangers of inadequate cosmetics regulations for women of color.
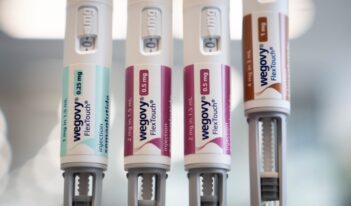
Regulating Weight-Loss Drugs and Supplements
Scholars discuss the regulatory landscape surrounding GLP-1 drugs and dietary supplements.
Drilling Down on Loper Bright and Health Care Regulation
The Loper Bright decision leaves hundreds of pivotal health care regulations subject to litigation.
Digital Health Technologies for an Aging Population
Scholars examine the challenges of using digital health technologies to care for cognitively impaired adults.
Creating a Culture of Compliance
Lauren Steinfeld discusses regulatory compliance in the context of data privacy and health care.
The Underregulation of Carcinogen Exposure
Scholar calls for Congress to empower regulators to limit exposure to cancer-causing chemicals.
Open Data, Closed Doors?
Better regulation can eliminate high-cost barriers to the integration of electronic health records.
Navigating Genetic Data Privacy and Law Enforcement Access
Scholars discuss the regulatory landscape surrounding genetic data privacy.
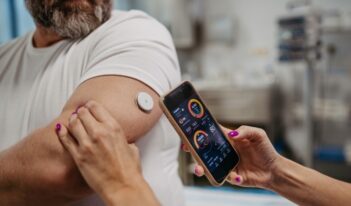
Reducing Barriers to Glucose Monitoring
FDA approves first over-the-counter continuous glucose monitor for U.S. patients.
The Past, Present, and Future of EMTALA
Scholars discuss EMTALA’s effects on physicians and argue for its continued use.
Forces that Shape and Constrain Medical Practice
Michelle Mello discusses how the law, artificial intelligence, and the COVID-19 pandemic have shaped health care.
Regulatory Collaboration to Protect Public Health
Noah Aleshire, chief regulatory officer at the CDC, discusses the value of a collaborative and informed regulatory process.