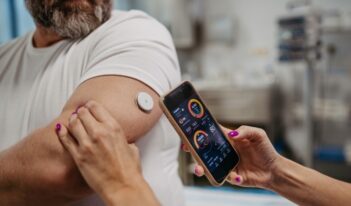
Reducing Barriers to Glucose Monitoring
FDA approves first over-the-counter continuous glucose monitor for U.S. patients.
Regulating Agricultural Water Quality
Contaminated agricultural water is a well-known root cause of foodborne illness that regulators have struggled to address.
FDA Targets Toxic Soda Ingredients
FDA proposes a rule that would prohibit the use of a toxic food additive in sodas.
Navigating Neurotechnological Regulations
Researchers address the challenges regulators face in protecting the public amid the rapid growth of neurotechnologies.
Regulating Off-Label Prescribing
Scholars and practitioners debate whether and how to regulate off-label drug use.
The Future of Plant-Based Meats
Scholar recommends that regulators change food label laws to increase consumers’ access to plant-based meats.
Challenging Drug Patents to Lower Prices
The FTC cracks down on improperly listed drug patents, challenging over 100 patent listings.
Why We Need To Talk About Psychedelic Dispensaries
Dispensaries could fill the regulatory void for psychedelics and promote public health.
A Roadmap to Reimbursement for Psychedelics
Insurance reimbursement for psychedelic therapy is integral to treatment accessibility.
Branching Regulatory Paths and Dead Ends in Psychedelics
The convergence of current psychedelics regulatory pathways may bring benefits and new challenges.
Administrative Law Essay Competition Winners
Two essays by the student winners of a Penn Carey Law essay competition describe important regulatory issues.