Taking Hearing Aids Over the Counter
FDA proposes a new rule to permit the purchase of hearing aids without a prescription.
Addressing the Dangers of Forever Chemicals
Experts discuss regulatory options for PFAS chemical contamination control.
Addressing the Medical Device Safety Crisis
Scholar calls on FDA to prioritize public health by reforming medical device regulation.
Cracking the Egg Donation Market
The booming U.S. egg donation industry requires more regulation to safeguard donor welfare.
Medical AI Regulators Should Learn from the Global Financial Crisis
In regulating AI in medicine, FDA should exercise care in implementing a principles-based framework.
Essentially Snake Oil
The structure of some essential oils companies makes it harder for federal agencies to restrain false claims.
Regulatory Sandboxes Slowed the Spread of COVID-19
Scholar claims that regulatory sandboxes such as emergency use authorizations boost innovation, but at a cost.
Formaldehyde Lurks in Hair Products Despite FDA Warning
FDA warns consumers to protect themselves from a known carcinogen in hair smoothing products.
Regulating Drugs at a Discount
Scholars examine the effectiveness of the contentious 340B Drug Pricing Program.
Discriminatory Blood Donation Policies Defy Science
COVID-19 highlights a blood donation deferral policy that clashes with modern science.
Something Fishy About Seafood Inspection
Government watchdog finds that FDA is not properly inspecting imported seafood.
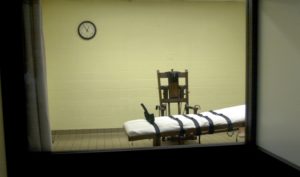
Making Drugs Safe to Kill?
Lawyer proposes a new legal framework for reviewing FDA’s refusal to regulate lethal injection drugs.